Slide 1 Home Next »

TEXT VERSION OF SLIDE:
Title: Introduction to Ionizing Radiation
Type: Text Slide
Content:
Bob Curtis
OSHA Salt Lake Technical Center
Supplement to Lecture Outline
V. 10.02
Slide 2 « Previous Next »
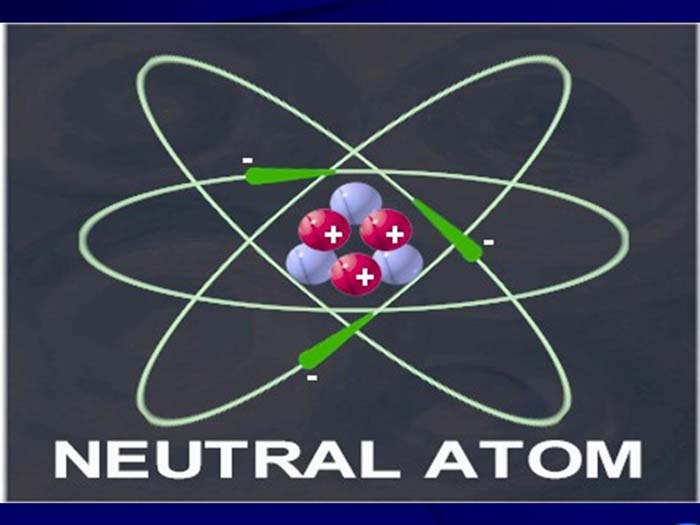
TEXT VERSION OF SLIDE:
Title: Neutral Atom
Type: Picture Slide
Content: [Includes image of a neutral atom]
Slide 3 « Previous Next »
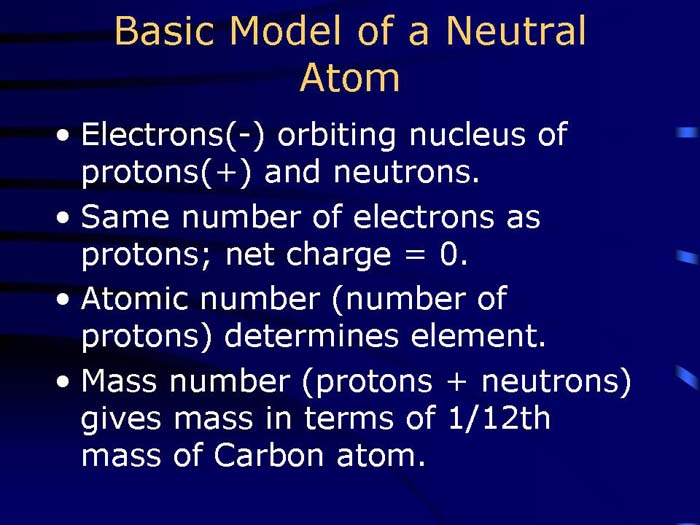
TEXT VERSION OF SLIDE:
Title: Basic Model of a Neutral Atom
Type: Text Slide
Content:
- Electrons (-) orbiting nucleus of protons (+) and neutrons.
- Same number of electrons as protons; net charge = 0.
- Atomic number (number of protons) determines element.
- Mass number (protons + neutrons) gives mass in terms of 1/12th mass of Carbon atom.
Slide 4 « Previous Next »

TEXT VERSION OF SLIDE:
Title: Ionization vs. Exitation
Type: Text Slide
Content:
- Excitation transfers enough energy to an orbital electron to displace it further away from the nucleus.
- In ionization the electron is removed, resulting in an ion pair.
- The newly freed electron (-) and the rest of the atom (+).
Slide 5 « Previous Next »
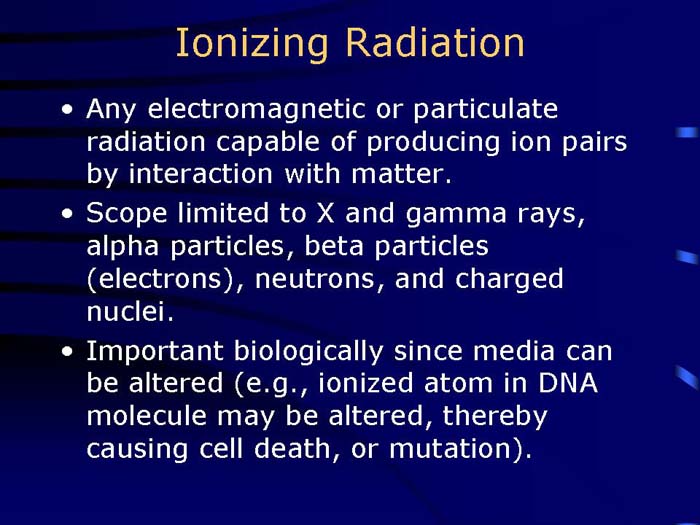
TEXT VERSION OF SLIDE:
Title: Ionizing Radiation
Type: Text Slide
Content:
- Any electromagnetic or particulate radiation capable of producing ion pairs by interaction with matter.
- Scope limited to X and gamma rays, alpha particles, beta particles (electrons), neutrons, and charged nuclei.
- Important biologically since media can be altered (e.g., ionized atom in DNA molecule may be altered, thereby causing cell death, or mutation).
Slide 6 « Previous Next »
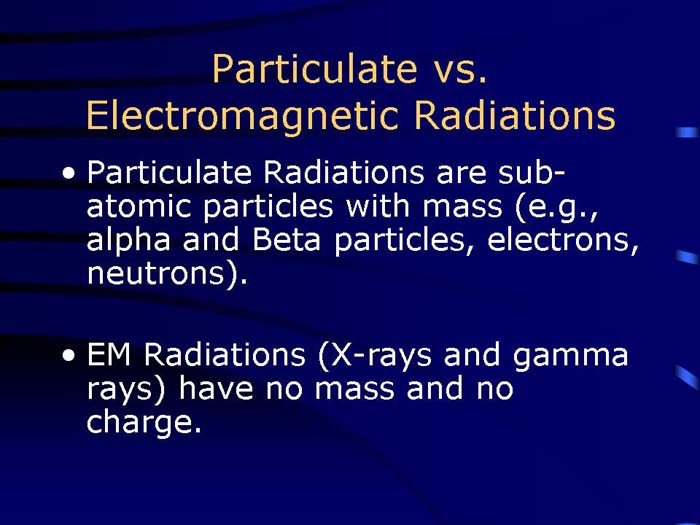
TEXT VERSION OF SLIDE:
Title: Particulate vs. Electromagnetic Radiations
Type: Text Slide
Content:
- Particulate Radiations are subatomic particles with mass (e.g., alpha and Beta particles, electrons, neutrons).
- EM Radiations (X-rays and gamma rays) have no mass and no charge.
Slide 7 « Previous Next »
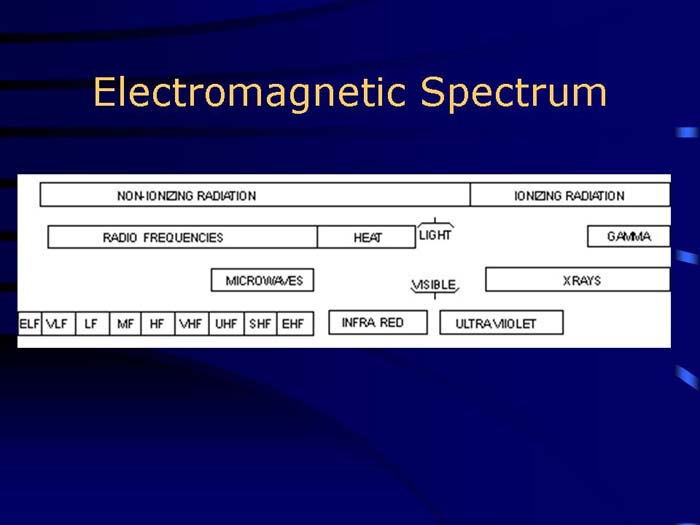
TEXT VERSION OF SLIDE:
Title: Electromagnetic Spectrum
Type: Picture Slide
Content: [Image of the electromagnetic spectrum.]
|
NON-IONIZING RADIATION
IONIZING RADIATION |
||||||||||||||
RADIO FREQUENCIES |
HEAT |
| / LIGHT \ |
GAMMA |
|||||||||||
MICROWAVES |
\ VISIBLE / | |
XRAYS |
||||||||||||
|
INFRA RED |
ULTRAVIOLET |
||||||||||||
Slide 8 « Previous Next »

TEXT VERSION OF SLIDE:
Title: High vs. Low Energy Radiation
Type: Text Slide
Content:
- Absorption of radiation is the process of transferring the energy of the radiation to the atoms of the media through which it is passing.
- Higher energy radiation of the same type will penetrate further.
- Usually expressed in KeV or MeV
- 1 eV = 1.6 x 10-19 Joules = 1.6 x 10-12 ergs
Slide 9 « Previous Next »
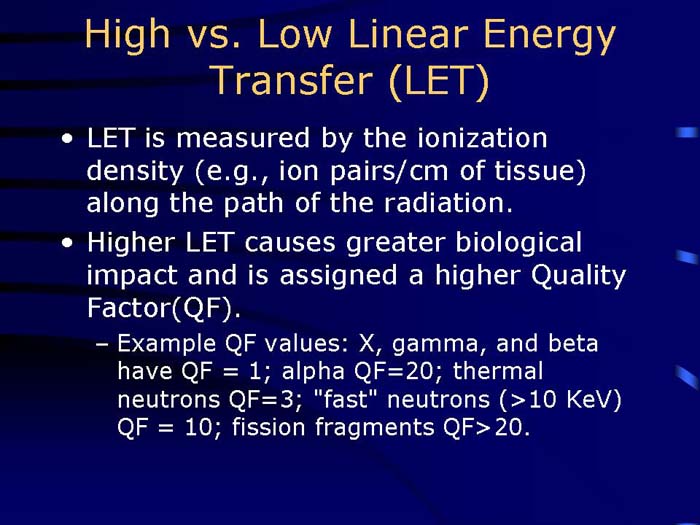
TEXT VERSION OF SLIDE:
Title: High vs. Low Energy Transfer (LET)
Type: Text Slide
Content:
- LET is measured by the ionization density (e.g., ion pairs/cm of tissue) along the path of the radiation.
- Higher LET causes greater biological impact and is assigned a higher Quality Factor (QF).
- Example QF values: X, gamma, and beta have QF = 1; alpha QF=20; thermal neutrons QF=3; "fast" neutrons (>10 KeV) QF = 10; fission fragments QF>20.
Slide 10 « Previous Next »
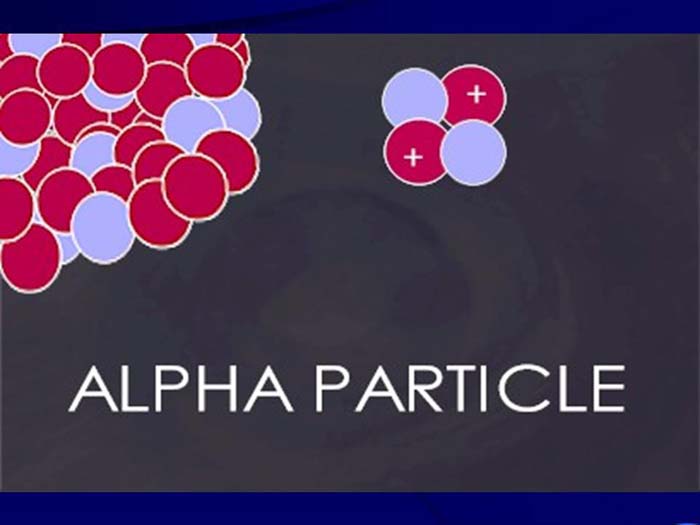
TEXT VERSION OF SLIDE:
Title: Alpha Particle
Type: Picture Slide
Content: [Includes image of alpha particle.]
Slide 11 « Previous Next »

TEXT VERSION OF SLIDE:
Title: Alpha Particles (or Alpha Radiation)
Type: Text Slide
Content:
- Helium nucleus (2 neutrons and 2 protons); +2 charge; heavy (4 AMU). Typical Energy = 4-8 MeV;
- Limited range (<10cm in air; 60µm in tissue);
- High LET (QF=20) causing heavy damage (4K-9K ion pairs/µm in tissue);
- Easily shielded (e.g., paper, skin) so an internal radiation hazard.
Slide 12 « Previous Next »
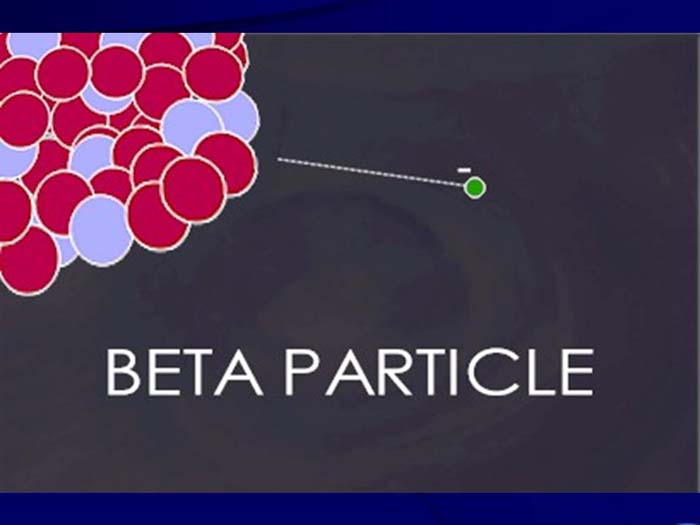
TEXT VERSION OF SLIDE:
Title: Beta Particle
Type: Picture Slide
Content: [Image of a beta particle.]
Slide 13 « Previous Next »

TEXT VERSION OF SLIDE:
Title: Beta Particles
Type: Text Slide
Content:
- High speed electron ejected from nucleus; -1 charge; light 0.00055 AMU; Typical Energy = several KeV to 5 MeV;
- Range approx. 12'/MeV in air, a few mm in tissue;
- Low LET (QF=1) causing light damage (6-8 ion pairs/µm in tissue);
- Primarily an internal hazard, but high beta can be an external hazard to skin.
Slide 14 « Previous Next »
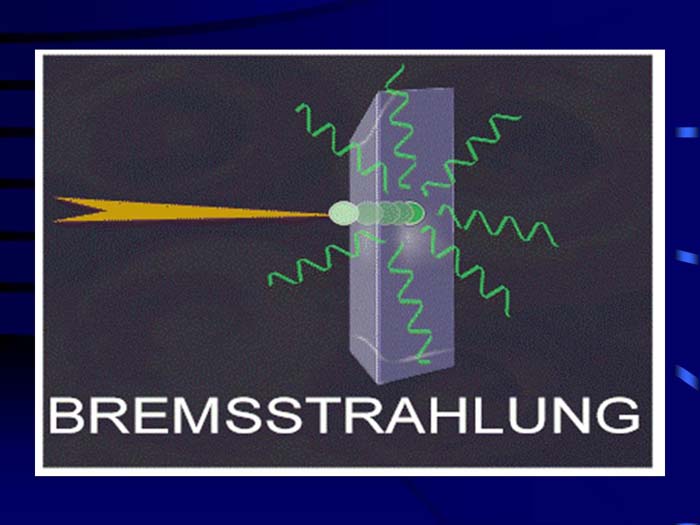
TEXT VERSION OF SLIDE:
Title: Bremsstrahlung
Type: Picture Slide
Content: [Includes image of bremsstrahlung.]
Slide 15 « Previous Next »

TEXT VERSION OF SLIDE:
Title: Bremsstralung (or Braking) Radiation
Type: Text Slide
Content:
- High speed electrons may lose energy in the form of X-rays when they quickly decelerate upon striking a heavy material.
- Aluminum and other light (<14) materials and organo-plastics are used for shielding.
Slide 16 « Previous Next »

TEXT VERSION OF SLIDE:
Title: Positrons
Type: Text Slide
Content:
- Beta particles with an opposite (+) charge.
- Quickly annihilated by combination wtih an electron, resulting in gamma radiation.
Slide 17 « Previous Next »

TEXT VERSION OF SLIDE:
Title: Neutrons
Type: Text Slide
Content:
- Neutrons ejected from a nucleus; 1 AMU; 0 Charge;
- Free neutrons are unstable and decay by Beta emission (electron and proton separate) with T½ of approx. 13 min;
- Range and LET are dependant on "speed": Slow (<10 KeV), "Thermal" neutrons, QF=3; and Fast (>10 KeV), QF=10.
Slide 18 « Previous Next »

TEXT VERSION OF SLIDE:
Title: Shielding Neutrons
Type: Text Slide
Content:
- Shielded in stages: High speed neutrons are "thermalized" by elastic collisions in hydrogenous materials (e.g., water, paraffin, concrete).
- The "hit" nuclei give off the excess energy as secondary radiation (alpha, beta, or gamma).
- Slow neutrons are captured by secondary shielding materials (e.g., boron or cadmium).
Slide 19 « Previous Next »
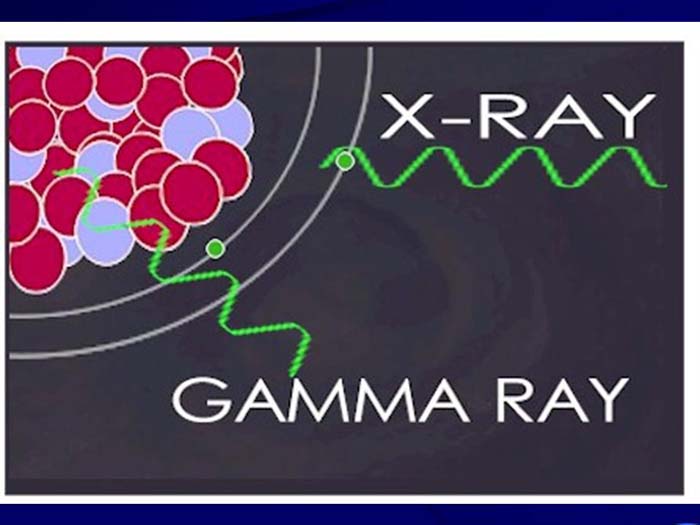
TEXT VERSION OF SLIDE:
Title: Gamma Ray
Type: Picture Slide
Content: [Includes image of a gamma ray and an x-ray.]
Slide 20 « Previous Next »
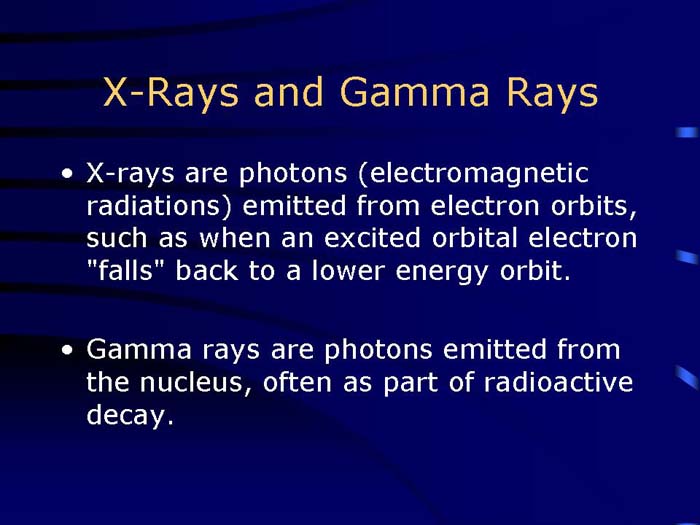
TEXT VERSION OF SLIDE:
Title: X-Rays and Gamma Rays
Type: Text Slide
Content:
- X-rays are photons (electromagnetic radiations) emitted from electron orbits, such as when an excited orbital electron "falls" back to a lower energy orbit.
- Gamma rays are photons emitted from the nucleus, often as part of radioactive decay.
Slide 21 « Previous Next »
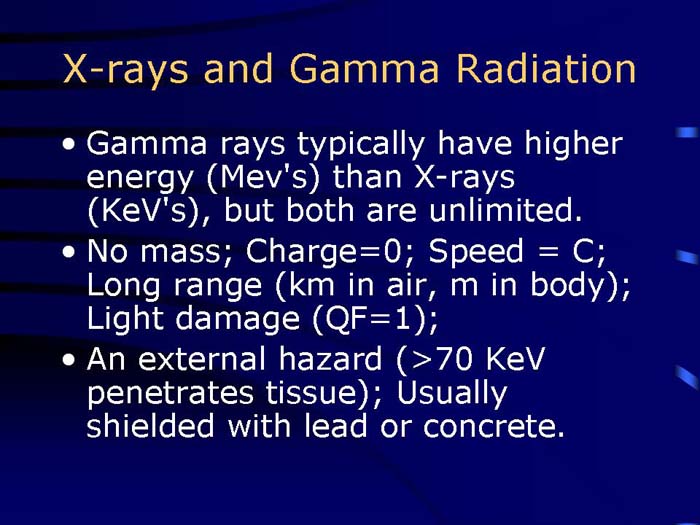
TEXT VERSION OF SLIDE:
Title: X-rays and Gamma Radiation
Type: Text Slide
Content:
- Gamma rays typically have higher energy (Mev's) than X-rays (KeV's), but both are unlimited.
- No mass; Charge=0; Speed = C; Long range (km in air, m in body); Light damage (QF=1);
- An external hazard (>70 KeV penetrates tissue); Usually shielded with lead or concrete.
Slide 22 « Previous Next »
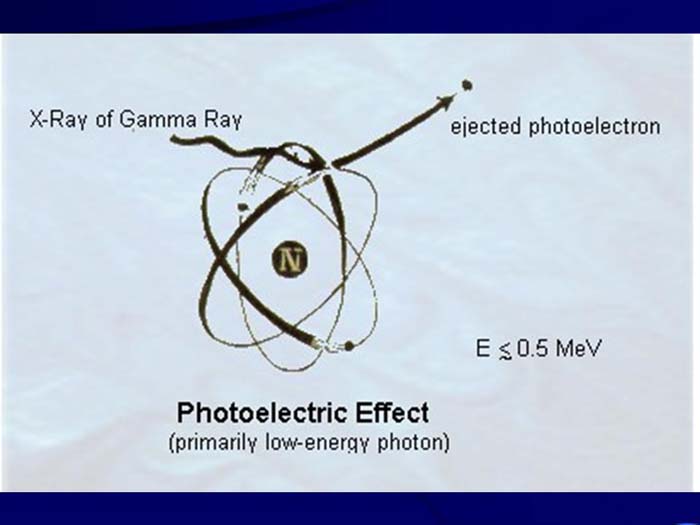
TEXT VERSION OF SLIDE:
Title: Photoelectric Effect
Type: Picture Slide
Content: [Includes illustration of the Photoelectric Effect (primarily low-energy photon). X-Ray of Gamma, ejected photoelectron and E ≲ 0.5 MeV.]
Slide 23 « Previous Next »
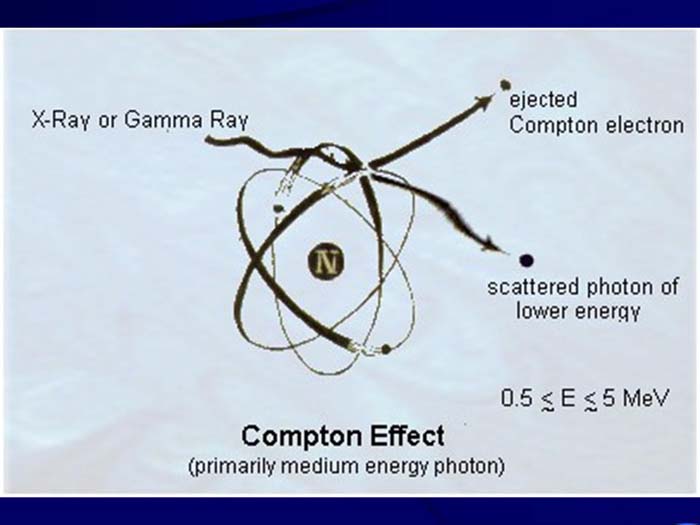
TEXT VERSION OF SLIDE:
Title: Compton Effect
Type: Picture Slide
Content: [Includes illustration of the Compton Effect (primarily medium energy photon). X-Ray or Gamma Ray, ejected Compton electron, scattered photon of lower energy, and 0.5 ≲ E ≲ 5 MeV.]
Slide 24 « Previous Next »
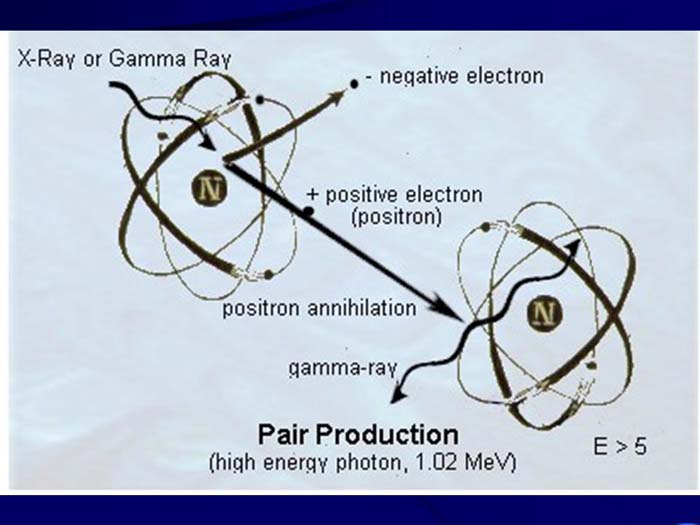
TEXT VERSION OF SLIDE:
Title: Pair Production
Type: Picture Slide
Content: [Includes illustration of a Pair Production (high energy photon, 1.02 MeV). X-Ray or Gamma ray, - negative electron, + positive electron (Positron), positron annihilation, E > 5.]
Slide 25 « Previous Next »

TEXT VERSION OF SLIDE:
Title: Radioactive Decay
Type: Text Slide
Content:
- Matter transforms from unstable to stable energy states.
- Radioactive materials are substances which spontaneously emit various combinations of ionizing particles (alpha and beta) and gamma rays of ionizing radiation to become more stable.
- Radioisotopes are isotopes (same number of protons but different numbers of neutrons) which are radioactive.
Slide 26 « Previous Next »
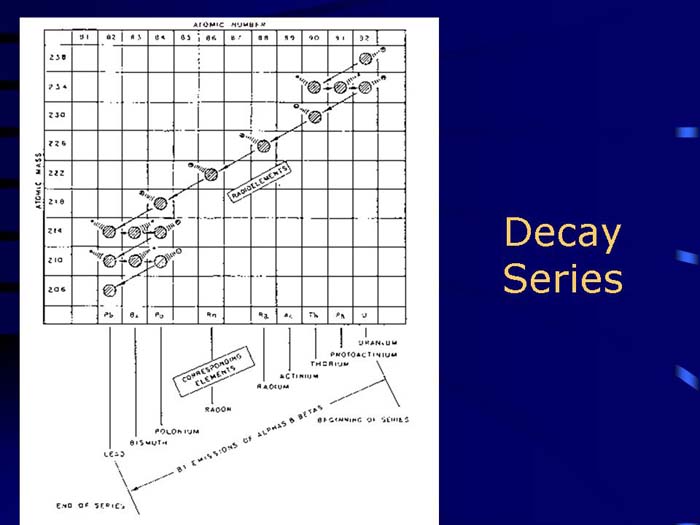
TEXT VERSION OF SLIDE:
Title: Decay Series
Type: Picture Slide
Content: [Includes illustration of a decay series.]
Slide 27 « Previous Next »

TEXT VERSION OF SLIDE:
Title: Equation Slide
Type: Picture Slide
Content:
Radium → alpha particle + Radon
Slide 28 « Previous Next »
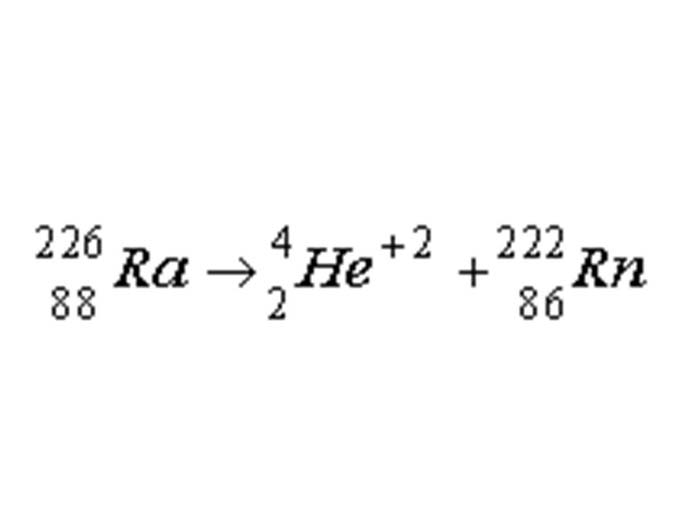
TEXT VERSION OF SLIDE:
Title: Equation Slide
Type: Picture Slide
Content:
| 226 | Ra → | 4 | He | +2 | + | 222 | Rn |
| 88 | 2 | 86 |
Slide 29 « Previous Next »
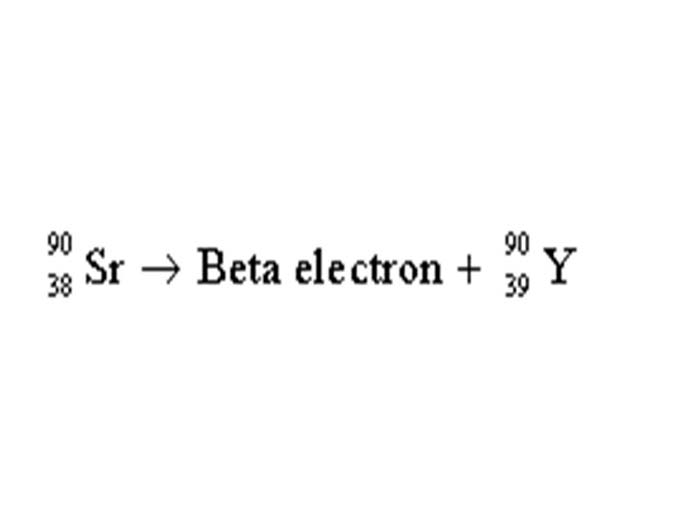
TEXT VERSION OF SLIDE:
Title: Equation Slide
Type: Picture Slide
Content:
| 90 | Sr → Beta electron + | 90 | Y |
| 38 | 39 |
Slide 30 « Previous Next »
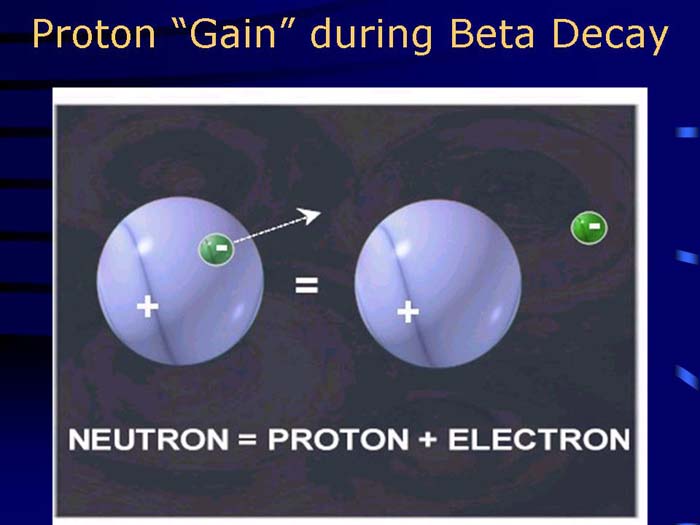
TEXT VERSION OF SLIDE:
Title: Proton "Gain" during Beta Decay
Type: Picture Slide
Content: [Image of Proton "Gain" during Beta Decay.]
Neutron = Proton + Electron
Slide 31 « Previous Next »
TEXT VERSION OF SLIDE:
Title: Beta Decay
Type: Text Slide
Content:
- No change in atomic mass; protons increase by 1.
- Consider a neutron as a proton enbedded with an electron; net charge = 0. When the electron is ejected, a proton is "created", thus increasing the atomic number.
Slide 32 « Previous Next »

TEXT VERSION OF SLIDE:
Title: Decay Series
Type: Text Slide
Content:
- Radioactive parent decays to a "daughter" which may also be radioactive, therefore, is also simultaneously decaying.
- Resulting exposure is to the combination of both decays (and possibly additional daughters).
- Radon daughters are an important example of series decay exposure in uranium mines and basements.
Slide 33 « Previous Next »
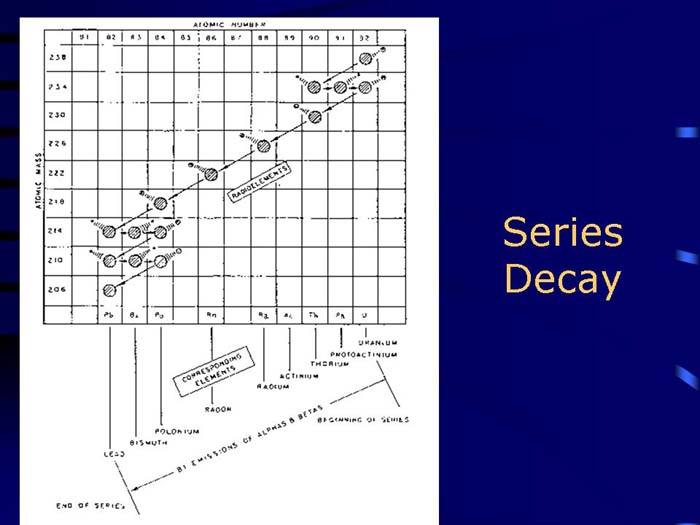
TEXT VERSION OF SLIDE:
Title: Series Decay
Type: Picture Slide
Content: [Includes illustration of a series decay.]
Slide 34 « Previous Next »
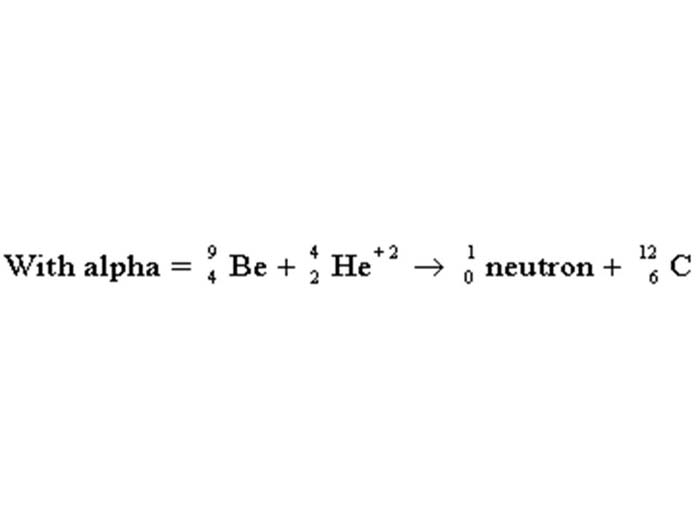
TEXT VERSION OF SLIDE:
Title:
Type: Picture Slide
Content:
| With alpha = | 9 | Be + | 4 | He+2→ | 1 | neutron + | 12 | C |
| 4 | 2 | 0 | 6 |
Slide 35 « Previous Next »

TEXT VERSION OF SLIDE:
Title: Monitoring equipment
Type: Picture Slide
Content: [Includes image of monitoring equipment.]
Slide 36 « Previous Next »
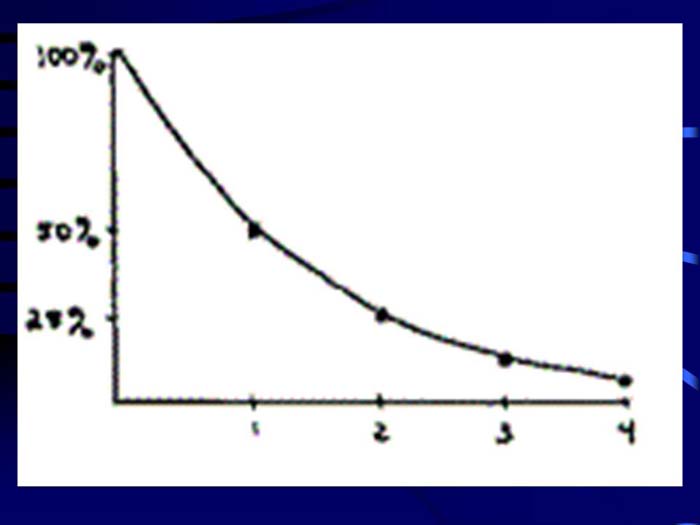
TEXT VERSION OF SLIDE:
Title: Sliding scale
Type: Picture Slide
Content: [Includes illustration of sliding scale. Estimated first point is 1 at 50%, 2 at 28%, 3 at 20% and 4 at 10%.]
Slide 37 « Previous Next »
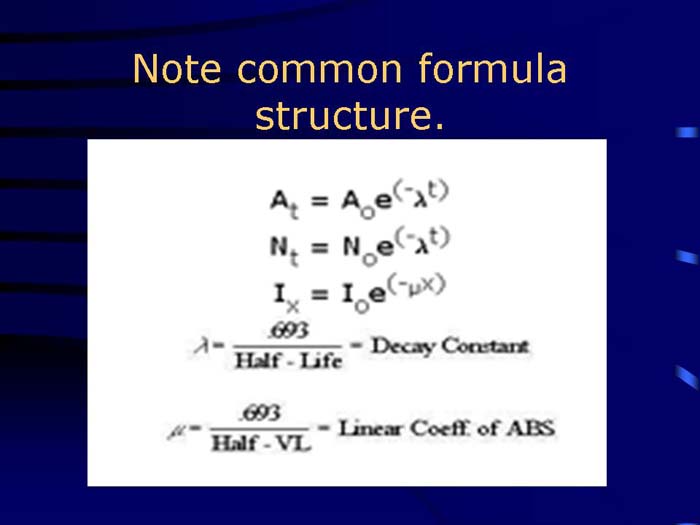
TEXT VERSION OF SLIDE:
Title: Note common formula structure.
Type: Picture Slide
Content: [Image of common formula]
| At = Aoe(-λt) | ||
| Nt = Noe(-λt) | ||
| Ix = Ioe(-µx) | ||
| λ = | .693 | = Decay Constant |
| Half - Life | ||
| µ = | .693 | = Linear Coeff. of ABS |
| Half - VL |
Slide 38 « Previous Next »
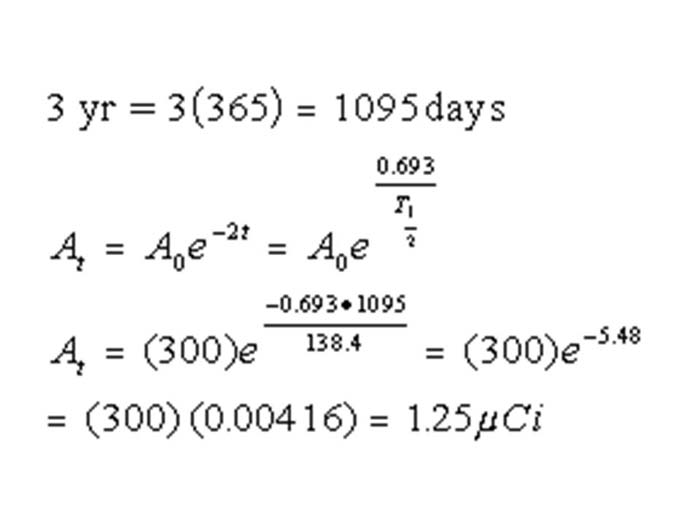
TEXT VERSION OF SLIDE:
Title: Equation
Type: Picture Slide
Content: [Image of an equation]
| 3 yr = 3(365) = 1095 days | |||
| 0.693 | |||
| At = A0e-2t = A0e | T½ | ||
| -0.693 • 1095 | |||
| At = (300)e | 138.4 | = (300)e-5.48 | |
| = (300)(0.00416) = 1.25µCi | |||
Slide 39 « Previous Next »
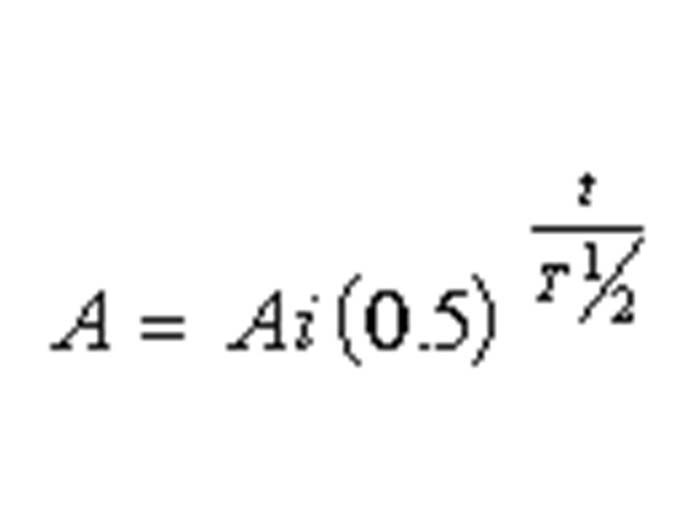
TEXT VERSION OF SLIDE:
Title: Equation
Type: Picture Slide
Content: [Image of an equation]
| A = Ai (0.5) | t |
| T½ | |
Slide 40 « Previous Next »
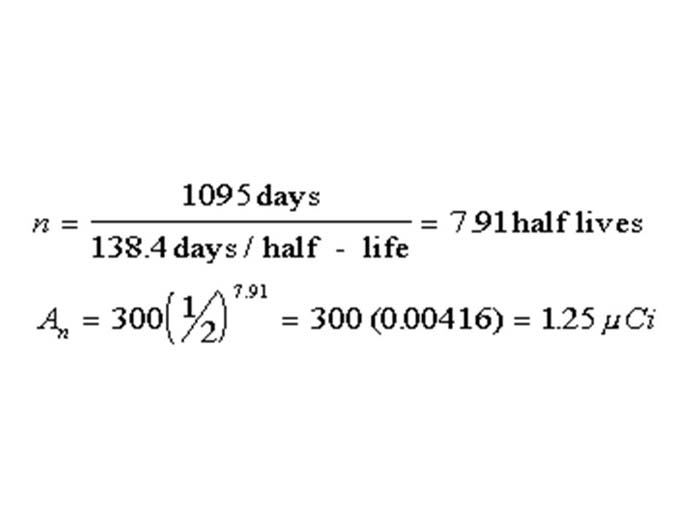
TEXT VERSION OF SLIDE:
Title: Equation
Type: Picture Slide
Content: [Image of an equation]
| n = | 1095 day | = 791 half lives |
| 138.4 days/half - life | ||
| An = 300 (½)7.91 = 300(0.00416) = 1.25µCi | ||
Slide 41 « Previous Next »
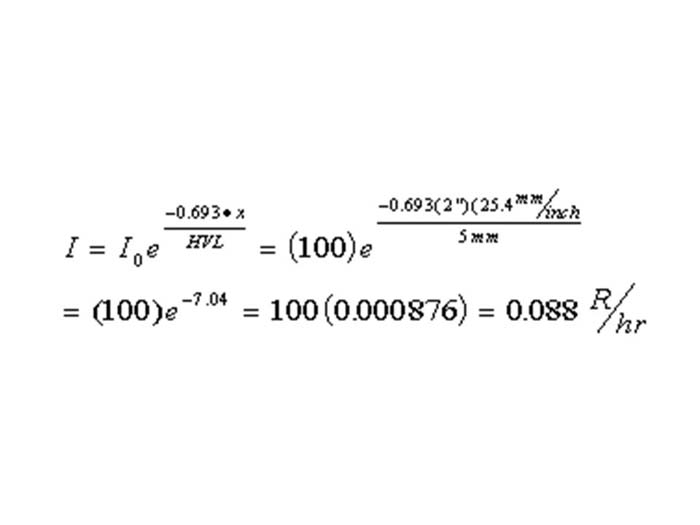
TEXT VERSION OF SLIDE:
Title: Equation
Type: Picture Slide
Content: [Image of an equation]
| I = I0e | -0.693 • x | = (100)e | -0.693(2")(25.4mm/inch | |||
| HVL | 5mm | |||||
| = (100)e-7.04 = 100(0.000876) = 0.088R/hr | ||||||
Slide 42 « Previous Next »

TEXT VERSION OF SLIDE:
Title: Calibration Source
Type: Picture Slide
Content: [Includes photo comparing the size of a quarter to a Eberline Cs-7a gamma radioactive material coin.]
Slide 43 « Previous Next »
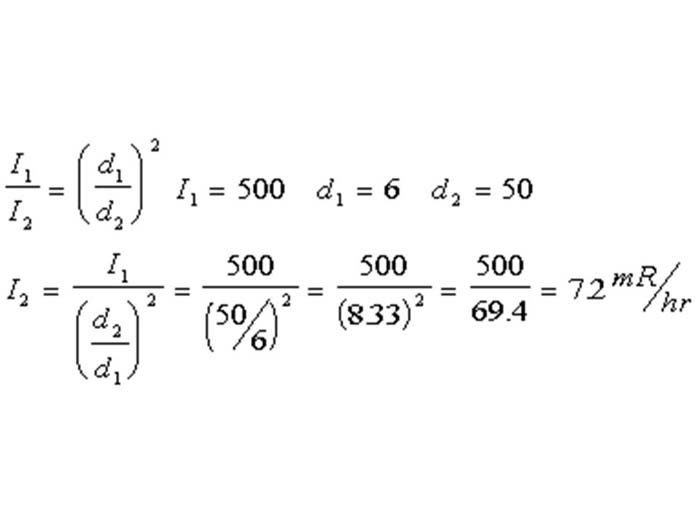
TEXT VERSION OF SLIDE:
Title: Equation
Type: Picture Slide
Content: [Image of an equation]
| I1 | = ( | d1 | )2 | I1 = 500 d1 = 6 d2 = 50 | |||||||||
| I2 | d2 | ||||||||||||
| I2 = | I1 | = | 500 | = | 500 | = | 500 | =7.2 m R/hr | |||||
| ( | d2 | )2 | (50/6) | 2 | (833)2 | 69.4 | |||||||
| d1 | |||||||||||||
Slide 44 « Previous Next »
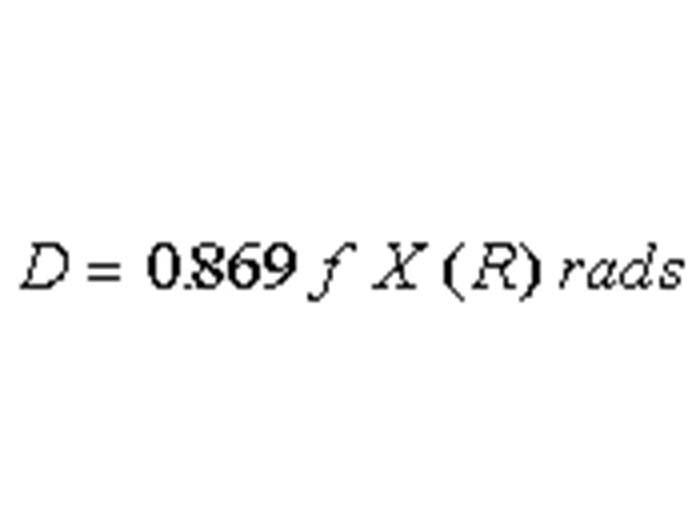
TEXT VERSION OF SLIDE:
Title: Equation
Type: Picture Slide
Content: [Image of an equation]
D = 0.869 ƒ X (R) rads
Slide 45 « Previous Next »
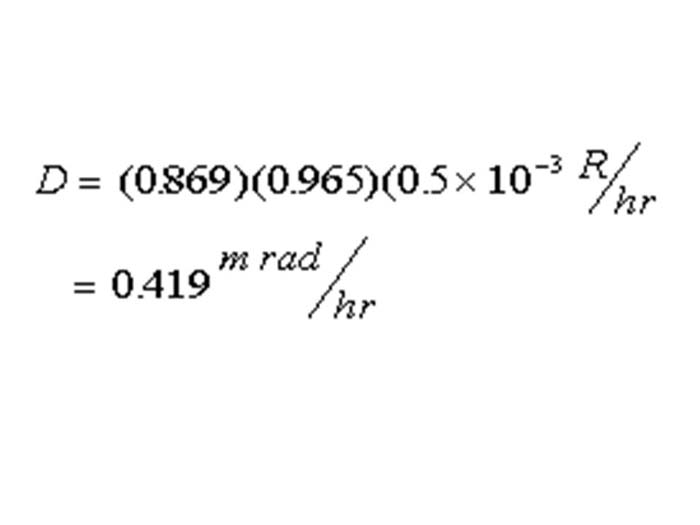
TEXT VERSION OF SLIDE:
Title: Equation
Type: Picture Slide
Content: [Image of an equation]
D = (0.869)(0.965)(0.5 x 10-3 R/hr
=0.419m rad/hr
Slide 46 « Previous Next »

TEXT VERSION OF SLIDE:
Title: Monitoring equipment
Type: Picture Slide
Content: [Includes image of monitoring equipment.]
Slide 47 « Previous Next »
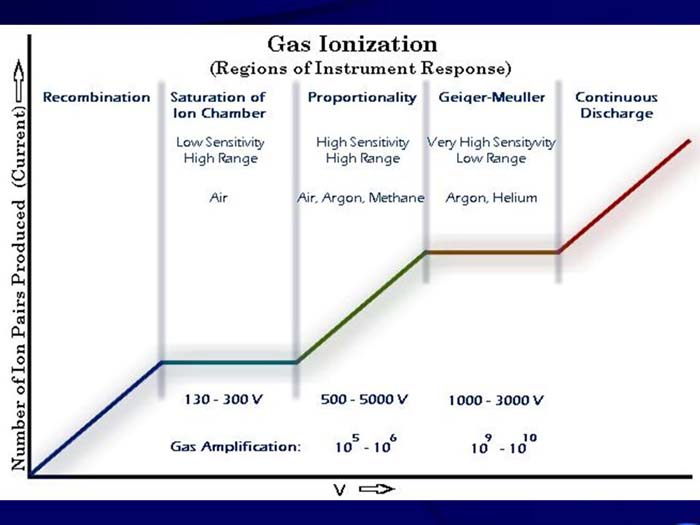
TEXT VERSION OF SLIDE:
Title: Gas Ionization (Regions of Instrument Response)
Type: Picture Slide
Content: [Includes illustration of gas ionization regions of instrument response categorized by number of ion pairs produced. Y-axis - Number of Ion Pairs Produced (Current) -->, X-axis - V -->.]
- 5 sections accross the x-axis.
- First - Recombination
- Second - Saturation of Ion Chamber {Low sensitivity, High Range, Air} 130 - 300 V
- Third - Proportionality {High Sensitivity High Range, Air, Argon, Methane} 500 - 5000 V Gas Amplification: 105 - 106
- Fourth - Geiqer-Mueller {Very High Sensityvity low Range, Argon, Helium} 1000 - 3000 V Gas Amplification: 109 - 1010
- Fifth - Continuous Discharge
Slide 48 « Previous Next »

TEXT VERSION OF SLIDE:
Title: Monitoring equipment
Type: Picture Slide
Content: [Image of a man using a Geiger Counter.]
Slide 49 « Previous Next »

TEXT VERSION OF SLIDE:
Title: Monitoring equipment
Type: Picture Slide
Content: [Includes image of monitoring equipment.]
Slide 50 « Previous Next »

TEXT VERSION OF SLIDE:
Title: Laboratory Equipment
Type: Picture Slide
Content: [Includes image of laboratory equipment.]
Slide 51 « Previous Next »

TEXT VERSION OF SLIDE:
Title: Laboratory Equipment
Type: Picture Slide
Content: [Includes image of laboratory equipment.]
Slide 52 « Previous Next »
TEXT VERSION OF SLIDE:
Title: Chemist Working in a Laboratory
Type: Picture Slide
Content: [Includes image of chemist working in laboratory.]
Slide 53 « Previous Next »

TEXT VERSION OF SLIDE:
Title: Chemical Storage
Type: Picture Slide
Content: [Includes image of chemical storage room.]
Slide 54 « Previous Home

TEXT VERSION OF SLIDE:
Title: Radioactive Symbols
Type:Picture Slide
Content: [Includes images of a radioactive symbols NFPA 704M Label and a D.O.T. Label.]